IFNANO-Technologie für STED-Mikroskopie-Systeme lizensiert
Göttingen, 4.7.2023
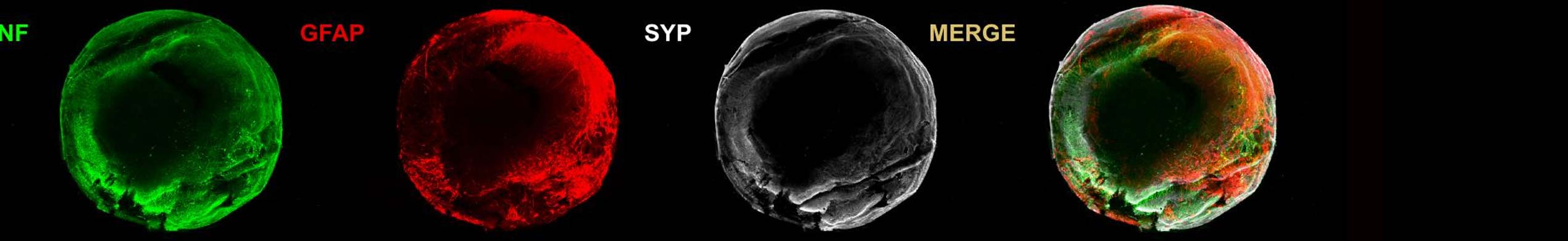
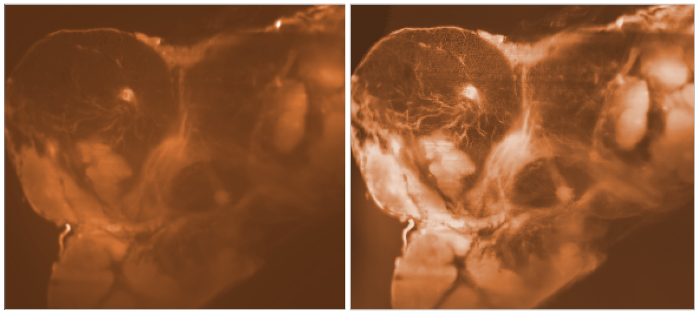
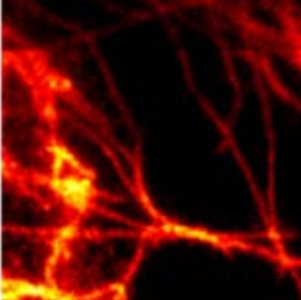
An dem Institut für Nanophotonik (IFNANO) wurde eine Methode entwickelt, welche die STED-Mikroskopie einfacher und zuverlässiger macht und die Aufnahme noch schärferer Bilder in lebenden Zellen erlaubt. Die MBM ScienceBridge GmbH hat für diese IFNANO-Technologie erfolgreich einen Lizenzvertrag mit Leica Microsystems, einem der größten und erfolgreichsten Mikroskopieunternehmen weltweit, vermittelt.
weiterlesen