Raman Refractometer Analyzer
The following invention describes methods and apparatus for identifying infusions when administered through a syringe pump. The method provides for a coupling of Raman-spectroscopy and refractometry. Both analysis methods used originate from the instrumental analytics. Furthermore, algorithms are used for the evaluation that are similar to Indirect Hard Modelling (IHM) and statistics.
Challenge
Incorrect administration of medications and infusion solutions continues to be a not insignificant problem in hospitals. drugs and infusion solutions. With the aid of a combination of Raman spectroscopy and refractometry, drug solutions can be clearly identified and incorrect medication can be and medical personnel can then intervene. When comparing the analysis results with the patient's file in the hospital's EDP system, additional data (e.g. the drug incompatibility) can trigger an alert to the staff. Current systems for reduction of medication errors are based on non-technical approaches such as the use of barcode systems. application of barcode systems.
Our solution

(Figure 1.: Study of an automated analyzer to identify the infusion solution in the syringe.)
The drugs, which are also present in solutions, among others, are analyzed Raman spectroscopically and refractometrically within a flow cell. The results of the two methods are combined and compared with an existing spectra and refractive index database for the drugs. This represents an initial result that is output by the automated analyzer. The output of the extended final result is output after comparison of the first result with the hospital EDP. Such a measurement strategy is made possible by the fact that many drugs are already detectable by Raman spectroscopy alone. However, aqueous solutions of NaCl and KCl cannot be distinguished in this way. At this point, analysis by refractometry takes place in a downstream process. In this way, the aqueous solutions of NaCl and KCl used in medicine can also be clearly distinguished from one another. In the next step, algorithms using IHM and statistical methods detect both the composition of the drugs in the solution and their quantities. The result of the Raman measurement allows qualitative and quantitative identification of the Raman-active substances. In this case, refractometry is used to verify the Raman result. The result of the quantification is optimized at the same time. For Raman-inactive substances, this is not possible directly. Here, the qualification and quantification is the responsibility of refractometry.
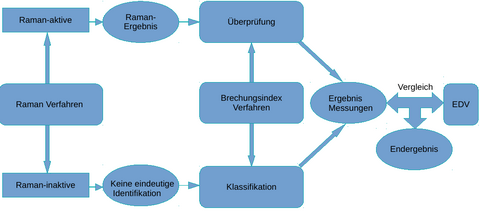
(Figure 2.: Schematic representation of data fusion and comparison with an internal database and optional comparison with hospital IT about the drug to be used.)
Advantages
- Direct analysis of medication
- Combination of Raman-spectroscopy and refractometry
- Detection of incorrect medication
- Reduction of human errors
Applications
- Medical field (hospitals, intensive care units) for monitoring infusions
- Petrochemical industry
- Food industry
- General possibility of quality control in liquids
Development Status
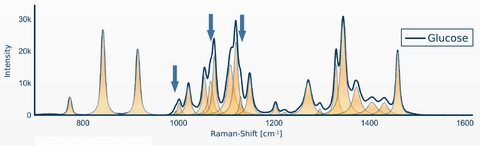
Figure 3 shows an example of Raman signal detection of glucose. In addition, automated detection of the following substances such as: Insulin, Norepinephrine, Heparin, NaCl, KCl, NaBic has already been successfully realized. A final prototype also exists. The next step is the development of a commercial device.
Patent Status
German Patent application: DE102017108120A1 (disclosed)
European Patent application: EP3610245A1 (disclosed)
US Patent application: US10871399B2 (granted)
Patent applicant:
Institut für Nanophotonik Göttingen e.V.
Contact
Dr. Maria Kamper
Patentmanger (Physics, Technology and Software)
E-Mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel.: +49 (0) 551 30 724 159
Reference: CPA-1980-LLG
www.sciencebridge.de
Tags: Measurement and analysis technology, Physics and Technology & Software
