Pentafluorocyclopropanation of drugs and pesticides
The evaluation of the pentafluorocyclopropyl group as a chemotype in crop protection and medicinal chemistry has been hampered in the past by the lack of suitable methodologies that enable the practical incorporation of this moiety into advanced synthetic intermediates. By synthesizing an unprecedented sulfonium salt, 5-(pentafluorocyclopropyl)-dibenzothiophenium triflate, and using it as a versatile reagent for photoinduced pentafluorocyclopropylation, a wide range of (hetero)arenes are enabled by a radical-mediated mechanism.
Challenge
Typical benefits derived from fluorine substitution in drug candidates are resistance towards oxidative metabolism, changes in lipophilicity dependent on (poly)fluorination patterns, and some degree of, often counterintuitive, conformational control. Furthermore, structurally challenging novel (poly)fluorinated motifs are often incorporated into structures of therapeutic interest. The evaluation of pentafluorocyclopropyl groups as chemotypes in crop protection and medicinal chemistry has been hampered in the past by the lack of appropriate methods to practically incorporate this group into advanced synthetic intermediates. Although the cyclopropyl ring is common in the structures of drug candidates, its pentafluoro counterpart (formally derived from the cyclization of the two terminal -CF3 units in HFIP) has remained absent from drug optimization campaigns.
Our Solution
Scientists at the Georg-August-University Göttingen have synthesized an sulfonium salt, 5-(pentafluoro-cyclopropyl)-dibenzothiophenium triflate, and using it as a versatile reagent for photoinduced pentafluorocyclopropylation.
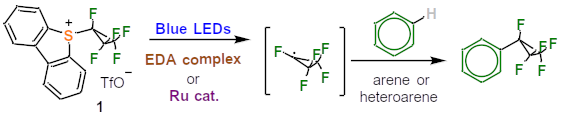 Fig.1 Synthesis of pentafluorocyclopropyl-substituted (hetero)arenes
Fig.1 Synthesis of pentafluorocyclopropyl-substituted (hetero)arenes
In particular, to enable electrophilic fluorocyclopropylation, the basic chemical structure of dibenzothiophene was used. To the Umemoto reagent, an organic compound developed in the 1990s and used for trifluoromethylation, the pentafluorocyclopropyl is attached to the dibenzothiophene instead of trifluoromethyl. The new reagents 5-(pentafluorocyclopropyl)dibenzothiophenium triflate and 5-(tetrafluorocyclopropyl)dibenzothiophenium triflate are formed as intermediates. This is followed by pentafluorocyclopropylation, i.e., transfer of the pentafluorocyclopropyl to (hetero)arenes by photoredox catalysis. Under photochemical conditions (irradiation with, for example, blue light diodes at a maximum wavelength of 462 nm) and in the presence of electron donor acceptor (EDA) complexes, a pentafluorocyclopropyl radical is generated, which ends up as a substituent in medical agents and biologically active molecules.
Advantages
- late stage functionalization of drugs and pesticides
- predictable site-selective installation of the pentafluorocyclopropane moiety at the innately most nucleophilic position(s) of many heterocyclic substrates
- operational ease of the experimental procedures
- light induced transfer
- two photocatalytic protocols that allows access to a broad array of unprecedented pentafluorocyclopropanated (hetero)arenes via C-H functionalization
- multi-gram scale synthesis
Applications
Late-stage functionalization of heterocyclic drugs and pesticides is of great value for the synthesis of fine chemicals in industry as well as for scientific and medical applications. Due to its ability to multi-fluorinate biologically relevant molecules and widely used drugs in the late stage of functionalization and its ease of application, it confers new properties to not previously functionalized (hetero)arenes through a radical-mediated mechanism.
Development Status
Successfull multi-gram scale synthesis achieved and photocatalytic protocols estabilished.
Patent Status
IP rights (DE102023117229.8) have been filed in the name of Georg-August-University of Göttingen and a licensing partner is sought.
References
Feng et al.: Pentafluorocyclopro-panation of (Hetero)arenes Using Sulfonium Salts: Applications in Late-Stage Functionalization. Angew Chem Int Ed Engl. 2023 Jul 4:e202306764. doi: 10.1002/anie.202306764
Contact
Dr. Vanessa Jensen
Patent Manager Life Sciences
E-Mail: Diese E-Mail-Adresse ist vor Spambots geschützt! Zur Anzeige muss JavaScript eingeschaltet sein!
Tel.: +49 551 30724 149
Referenz: BioV-2457-SUG
